Bevatas 100mg (Bevacizumab)
Препарат Bevatas 100 mg содержит Бевацизумаб — моноклональное антитело, направленное против VEGF (фактора роста эндотелия сосудов). Он подавляет образование новых кровеносных сосудов, питающих опухоль, и тем самым замедляет её рост и распространение. Применяется при колоректальном раке, немелкоклеточном раке лёгкого, раке яичников и других злокачественных опухолях в составе комбинированной терапии. Вводится внутривенно в дневном стационаре. Требует контроля артериального давления и наблюдения за риском кровотечений, перфорации кишечника и тромбозов.
Препарат Bevatas 100 mg содержит Бевацизумаб — рекомбинантное человеческое моноклональное антитело, которое избирательно связывается с VEGF и предотвращает его взаимодействие с рецепторами на эндотелиальных клетках. Это блокирует ангиогенез — ключевой механизм, обеспечивающий рост и метастазирование опухоли.
ПОКАЗАНИЯ
— Метастатический колоректальный рак (в комбинации с химиотерапией)
— Немелкоклеточный рак лёгкого (локально-распространённый или метастатический)
— Эпителиальный рак яичников, фаллопиевых труб или брюшины
— Глиобластома у взрослых при первичной или рецидивирующей форме
ВАЖНО
Бевацизумаб может вызывать серьёзные осложнения: носовые, лёгочные или желудочно-кишечные кровотечения, перфорацию кишечника, артериальные и венозные тромбозы, стойкую артериальную гипертензию и протеинурию. Противопоказан при активном кровотечении, в течение 4–6 недель после операции и при неконтролируемой гипертензии.
РЕЖИМ ДОЗИРОВАНИЯ
— При колоректальном раке: 5 мг/кг каждые 2 недели или 7,5 мг/кг каждые 3 недели
— При раке яичников: 15 мг/кг каждые 3 недели
— Флакон Bevatas 100 mg удобен для точного дозирования у пациентов с низкой массой тела
ПРОТИВОПОКАЗАНИЯ
— Гиперчувствительность к бевацизумабу
— Активное кровотечение или угроза перфорации ЖКТ
— Неконтролируемая артериальная гипертензия
— Недавний инсульт, ИМ или хирургическое вмешательство (менее 4–6 недель)
ПОБОЧНЫЕ ЭФФЕКТЫ
Часто: гипертензия, утомляемость, головная боль, протеинурия.
Серьёзно: геморрагия, перфорация кишечника, тромбоэмболия, задержка заживления ран.
ОСОБЫЕ УКАЗАНИЯ
Перед операцией терапию приостанавливают минимум за 4–6 недель. Женщины детородного возраста — обязательная контрацепция. При появлении крови в кале, рвоты с кровью или острой боли в животе — немедленно обратиться за медицинской помощью.
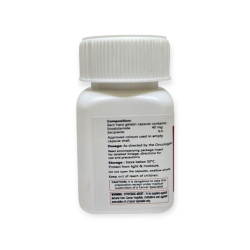
УСЛОВИЯ ХРАНЕНИЯ
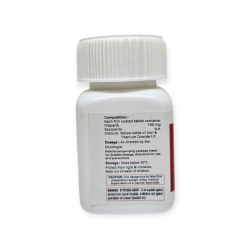
Хранить в холодильнике при 2–8°C, в оригинальной упаковке, не замораживать, оберегать от света.
| Фирменное наименование | Bevatas 100 mg |
| Действующее вещество | Бевацизумаб |
Пока нет комментариев