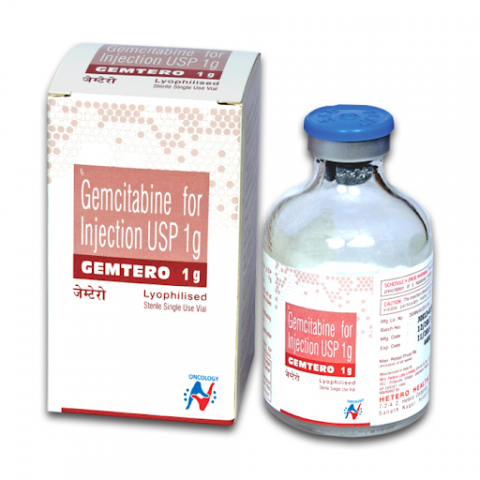
Gemtero 1g (Gemcitabine)
Gemtero 1g is a drug which is :
- Indicated for the treatment of Pancreas cancer
- Indicated for the treatment with combination of cisplatin for Non-small cell lung cancer
- Indicated for the treatment with combination of cisplatin for Bladder cancer
- Indicated for the treatment Soft-tissue sarcoma
- Indicated for the treatment with combination of paclitaxel for Metastatic breast cancer
- Indicated for the treatment with combination of carboplatin for Ovarian cancer
DESCRIPTION
Gemcitabine drug also represented by its brand name, Gemtero 1g which is a chemotherapy drug doctors classify as an antimetabolite, a group of drugs which reduce the growth of mesothelioma by prohibiting the proliferation of cancer cells and tumor expansion.
Gemtero 1g is a prescription drug which is used under the supervision of medical oncologist
INDICATION
Gemtero 1g is a drug which is :
- Indicated for the treatment of Pancreas cancer
- Indicated for the treatment with combination of cisplatin for Non-small cell lung cancer
- Indicated for the treatment with combination of cisplatin for Bladder cancer
- Indicated for the treatment Soft-tissue sarcoma
- Indicated for the treatment with combination of paclitaxel for Metastatic breast cancer
- Indicated for the treatment with combination of carboplatin for Ovarian cancer
MECHANISM OF ACTION
Tumors form when common cells become cancerous and initiate growing out of control. Normally, a healthy cell will split into a certain number of times, then go through apoptosis, pre-programmed cell death. Cancer cells bypass apoptosis and continue to multiply until they form a tumor.
Gemcitabine blocks the proliferation of rebellious cancer cells by prevent their production of DNA and RNA, the building stops the new cells. Lacking DNA and RNA, a cancer cell can’t regenerate new cells, and undergoes apoptosis.
ADME
By infusion length the volume of distribution is increased Vd: 50 L/m2
Metabolism transformed through nucleoside kinases to two active metabolites such as gemcitabine diphosphate and gemcitabine triphosphate The drug is excreted through urine (92-98%) and Half-life of Gemtero 1g is 1.7-19.4 hr
DOSAGE MANAGEMENT
Pancreatic cancer:
The usual dose is 1000mg/m2 IV infusion over 30 min once weekly x 7 weeks; rest 1 week
And the dose 1000mg/m2IV once weekly for 3 weeks of each 28-day cycle
Non-small cell lung cancer:
The usual dose is 1000mg/m2IV infusion over 30 minutes on days 1,8 and 15 of each 28-day cycle or The usual dose is 1250 mg/m2IV over 30 minutes on days 1 and 8 of each 21-day cycle
Administer cisplatin 100mg/m2 IV after gemcitabine on day 1
Breast cancer:
The usual dose is 1250 mg/m2IV over 30 minutes on days 1 and 8 of each 21-day cycle
Dose with paclitaxel 175mg/m2 on day 1 as a 3-hr infusion before gemcitabine
Ovarian cancer:
The usual dose is 1000 mg/m2IV over 30 minutes on days 1 and 8 of each 21-day cycle.
Dose with carboplatin AUC 4 on day 1 after gemcitabine
PRECAUTIONS
- When treatment a syndrome where fluid from your veins can leak into your tissues and leads a reduction in your blood pressure and fluid to deposited in your tissues and/or lungs
• By Gemtero 1gusage kidney function changes are reported, which can cause kidney failure • By Gemtero 1g usage Liver function changes are reported, which can cause liver failure and may be life-threatening
• When treatment with Gemtero 1g will leads to Inflammation (swelling) of the lungs and/or thickening of the lung tissues, which may be life-threatening. You may have a dry cough or trouble breathing.
• Haemolytic uremic syndrome resulted, containing fatalities; permanently stop treatment in patients with HUS or serious kidney impairment; Kidney failure may not be reversible even with stop of therapy.
SIDE EFFECTS
- Abnormal results in liver function tests.
• Blood in the urine
• Sleepiness
• Mouth ulcers.
• Diarrhoea or constipation.
• Cough.
• Blocked or runny nose.
• Sweating.
• Shortness of breath
• Alopecia
• Allergic skin rash
• Cold or flu-like symptoms.
• Retention of fluid in the body
• Back pain.
• Myalgia
• Loss of appetite.
• Headache.
• Insomnia
• Chills and fever.
• Weakness
DRUG INTERACTION
No drug interaction studies have been conducted
CONTRAINDICATIONS
Gemtero 1g is contraindicated in patients with known Hypersensitivity
PREGNANCY
The Gemtero 1g Pregnancy category D
When administrated to a pregnant woman will cause fetal harm to the fetus the pregnant women should be apprised of the possible hazard to a fetus.
LACTATION
Avoid breastfeed during this treatment because the drug Gemtero 1g may excreted into breast milk
STORAGE
The drug Gemtero 1g Store at 20°C to 25°C (68°F to 77°F)
MISSED DOSE
If failed to take dose, then have the missed dose as soon as possible before next dose time reaches or skip the dose and follow the normal schedule
Consult the doctor
Пока нет комментариев