Zelgor (Abiraterone Acetate )
Zelgor is belongs to anti neoplastic drugs which will work by Diminish the androgen production in the body. Androgen is a male hormone which balances the cancer cell in prostate glands.
DESCRIPTION
Zelgor is belongs to anti neoplastic drugs which will work by Diminish the androgen production in the body. Androgen is a male hormone which balances the cancer cell in prostate glands.
Normaly this tablet is taken by combination with prednisolone, a steroid which decreases the liability of side effects produced by Abiraterone.
MECHANISM OF ACTION
Androgens are regulates the production of tumors in prostate. Zelgor is an anti androgen drugs, which causes to eradicate the enzyme 17α-hydroxylase C 17, 20-lyase CYP17), it is includes in androgen synthesis. Decrease the serum testosterone level. Diminished the tumor cell occurred.
PHARMACOKINETIC
Absorption
Time to median plasma concentration of tablet Zelgor takes 12 hours.
Distribution
Maximum bound to the human plasma protein like albumin and alpha-1 acid glycoprotein.
Metabolism
The main two metabolites of Abiraterone in human plasma are Abiraterone sulphate and N-oxide Abiraterone sulphate.
Elimination
Terminal half life of tablet Zelgor is 12 ± 5 hours. The drug mainly excreted in 88% feces and 5% in urine.
DOSAGE MANAGEMENT
The usual dose of Zelgor is endorsed as 4 tablets orally once daily The drug Zelgor given with 5mg prednisolone twice daily in empty stomach. Zelgor Tablets administrated on an empty stomach. No food should be consumed for at least 2 hours before and at least 1 hour after the dose of Zelgor Tablets is taken. Zelgor should be swallowed whole with water. Do not crush or chew the tablets.
OVERDOSAGE
Zelgor has no specific antitoxin, If overdose occurs stop the drug and provide general supportive measures such as monitoring arrhythmias and cardiac failure and assess liver function.
SIDE EFFECTS
Common effects
Fatigue, Arthralgia, Hypertension, Nausea, edema, Hypokalemia, Diarrhea, vomiting, Cough, headache, Glucocorticoid deficiency, Mineral corticoid deficiency Hepatotoxicity Hypertension, Decrease in ka level Adrenocortical insufficiency
Musculo skeletal and Connective tissue disorders
Joint swelling, discomfort, muscle discomfort
General Disorder
Oedema
Cardiac disorders
Hot flush, hypertension, arrhythmias, chest pain, cardiac failure
GIT disorders
Diarrhea, dyspepsia
Infections and Infestation
UTI
Pulmonary disorder
Cough
Renal
Urinary frequency, nocturia
Injuries
Fractures
DRUG INTERACTION
Zelgor interaction with dextromethorphan will increases the Cmax and AUC of dextromethorphan Zelgor interaction with strong CYP3A inhibitors or inducers , use combination with caution because pharmacokinetics is not evaluated.
PRECAUTION
If patient having any allergy, severe liver problem, or pregnant, Zelgor tablet should be discontinued Do not take other medications, i.e prescription, over-the-counter, vitamins, herbal remedies, etc be sure to inform the doctors before the treatment. Aspirin do not administering and products containing aspirin till doctor particularly allow the drug.
PREGNANCY
pregnancy category X (leads to fetal death): During treatment if women become pregnant, immediately stop the drug and give counseling to the women. Zelgor tablet is not recommended for women.
LACTATION
Zelgor is not used in women excretion in human milk is unknown and the drug effects in the nursing infant is unknown.
STORAGE
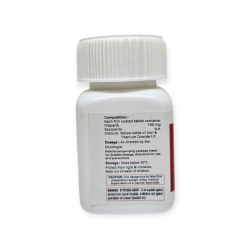
Stored at 15°C-30°C in a dry place.
MISSED DOSE
If patient missed the dose of Zelgor, that missed dose will be skipped and follow the regular dosing schedule.
Пока нет комментариев