Alectinib 150mg (Alectinib)
Препарат Alectinib 150 mg содержит Алектиниб — высокоселективный ингибитор тирозинкиназы ALK (анапластической лимфомы). Назначается при ALK-позитивном немелкоклеточном раке лёгкого (НМРЛ) у взрослых, включая пациентов с метастазами в головной мозг. Обладает высокой проницаемостью через гематоэнцефалический барьер, обеспечивая эффективный контроль церебральных поражений. Используется как в первой, так и во второй линии терапии. Требует подтверждения ALK-статуса опухоли до начала лечения.
ОСНОВНЫЕ СВОЙСТВА
Препарат Alectinib 150 mg содержит Алектиниб — оральный, высокоселективный и мощный ингибитор ALK (анапластической лимфомы тирозинкиназы). Он блокирует сигнальные пути, стимулирующие рост и выживание опухолевых клеток с транслокацией ALK. Отличается высокой активностью в ЦНС и благоприятным профилем безопасности по сравнению с более ранними ингибиторами (например, кризотинибом).
ПОКАЗАНИЯ
— Лечение взрослых с локально-распространённым или метастатическим ALK-позитивным немелкоклеточным раком лёгкого
— Применяется как в первой линии, так и после прогрессирования на фоне терапии кризотинибом
ВАЖНО
Обязательно подтверждение транслокации ALK (методом FISH, IHC или NGS) перед началом терапии. Без молекулярного подтверждения препарат неэффективен. Алектиниб может вызывать брадикардию, гепатотоксичность, микротрещины костей и отёки. Требуется контроль ЧСС, функции печени и кальция/витамина D.
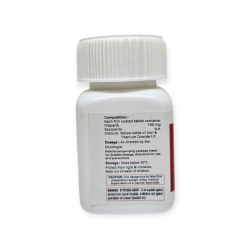
РЕЖИМ ДОЗИРОВАНИЯ
— Стандартная доза: 600 мг 2 раза в сутки (4 таблетки Alectinib 150 mg утром и вечером)
— Принимать с едой (повышает биодоступность)
— При токсичности — доза может быть снижена до 450 мг или 300 мг 2 раза в сутки
ПРОТИВОПОКАЗАНИЯ
— Гиперчувствительность к алектинибу
— Тяжёлая печёночная недостаточность (осторожность)
— Беременность и лактация
ПОБОЧНЫЕ ЭФФЕКТЫ
Часто: запор, утомляемость, отёки, миалгия, повышение АЛТ/АСТ.
Серьёзно: гепатотоксичность, брадикардия, интерстициальная болезнь лёгких, фотосенсибилизация, микротрещины костей.
ОСОБЫЕ УКАЗАНИЯ
Перед началом — ЭКГ, функция печени, уровень кальция/фосфора/витамина D. Избегать прямого солнечного света. При брадикардии — мониторинг ЧСС. Женщинам детородного возраста — надёжная контрацепция.
УСЛОВИЯ ХРАНЕНИЯ
Хранить в сухом месте при температуре до 30°C, в оригинальной упаковке, в недоступном для детей месте.
| Brand name | Alectinib 150 mg |
| Active substance | Алектиниб |
There are no comments yet