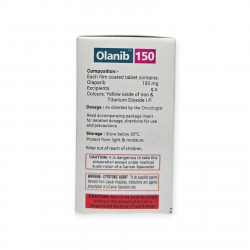
Bortenat 3.5mg (Bortezomib)
Bortenat 3.5mg is indicated to treat the patients suffering from multiple myeloma. Mantle cell lymphoma Bortenat 3.5mg is indicated to treat the disease mantle cell lymphoma which the patients have received at least 1 prior therapy.
DESCRIPTION
Bortezomib is sold under the brand name Bortenat 3.5mg which is a type of anti-cancer medicine, which is in the form of lyophilized powder A first beneficial proteasome inhibitor is known as Bortenat 3.5mg ; proteasome is a cellular structure which breaks the proteins.
Bortenat 3.5mg injection will helps to prevent from multiple myeloma in patients with or without before treatment history and mantle cell lymphoma.
INDICATION
Multiple myeloma Bortenat 3.5mg is indicated to treat the patients suffering from multiple myeloma. Mantle cell lymphoma Bortenat 3.5mg is indicated to treat the disease mantle cell lymphoma which the patients have received at least 1 prior therapy.
MECHANISM OF ACTION
Bortenat 3.5mg injection used in mammalian cells is a reversible inhibitor of the chymotrypsin-like activity of the 26-S proteasome. The drug prohibits targeted proteolysis that affects multiple signaling cascades responsible for normal homeostatic functions within the cell, leading to cell death.
ADME
ABSORPTION
The peak plasma concentration after 1st dose is 57 and 112ng/mL, If administrated two times a week, Cmax observed for 1mg/m2dose is 67 to 106ng/mL, for 1.3mg/m2 is 89 to 720ng/mL.
DISTRIBUTION
human serum plasma concentration is >80%
METABOLISM
Bortenat 3.5mg injection metabolized mainly through cytochrome P450 isoenzymes CYP3A4, CYP2C19 & CYP1A2.
ELIMINATION
The excretion pathways of Bortenat 3.5mg have not been characterized. The terminal elimination of half life after the 1mg/m2 dose is 40hrs - 193hrs, after dose 1.3mg/m2 is 76hrs - 108hrs.
DOSAGE MANAGEMENT
BORTENAT 3.5MG DOSAGE AND ADMINISTRATION
Bortenat is administrated alone or combination with dexamethasone Three week course is considered as therapy cycle Atleast 72 hours must slip away between constant doses of Bortenat. Bortenat (lyophilized powder form) is reconstituted by using 0.9% sodium chloride (NS) Bortenat should be reconstituted in 3.5ml NS The route of administration is IV bolus Intrathecal not use for administration In adults:
MANTLE CELL LYMPHOMA
The Bortenat recommended dose for untreated mantle cell lymphoma is;1.3mg/m2 of Bortenat administered as IV bolus given as two times weekly by combining with rituximab, cyclophospahmide, doxorubicin and tablet prednisolone for two weeks (day I, IV, VIII & XI) followed rest period of 10 days (day II through 21). In Relapse stage: The normal dose of Bortenat is 1.3mg/m2 administered as IV bolus or subcutaneous two times for two weeks (day I, IV, VIII & XI) followed by 10 day rest period (day II through 21) The therapy should followed for above 8 cycles may be given for once weekly for 4 weeks (day 1, 8, 15 & 22), followed by 13 day rest (days 23 through 35)
MULTIPLE MYELOMA
In the therapy of previously untreated multiple myeloma:1.3mg/m2 of dose must be given as 3 to 5 seconds through IV bolus or subcutaneous by concomitant use with tablet melphalan and prednisolone for nine 6 weeks therapy cycles Cycles 1 over 4, Bortenat is administrated for two times weekly, (day 1, 4, 8, 11, 22, 25, 29 and 32) Cycles 5 over 9, Bortenat is administrated once a week (day 1, 8, 22, and 29)
KEY POINTS
Moderately, 72 hours should be passing between following doses of Bortenat 3.5mg
IN RELAPSE STAGE
The usual dose of Bortenat is 1.3mg/m2 should be administered through IV bolus or subcutaneous as two times for two weeks (day1, 4, 8, and 11) continued by a ten day of rest course (day 12 through 21) The schedule of the treatment has been raised above 8 cycles may be taken once weekly for 4 weeks ( day 1, 8, 15, and 22), followed by 13 day rest (day 23 over 35)
PRECAUTIONS
While taking Bortenat 3.5mg some adverse effects occurs care should be taken in the conditions like Bortenat 3.5mg leads to peripheral neuropathy like burning sensation, hyperesthesia, hypoesthesia, paresthesia, neuropathic pain Manage postural Hypotension by altering the antihypertensive agents, hydration, and administration of mineralocorticoids or sympathomimetics Cardiac toxicity Pulmonary toxicity like Acute Respiratory Distress Syndrome, pneumonitis, interstitial pneumonia, lung infiltration Posterior reversible encephalopathy syndrome-stop the Bortenat 3.5mg therapy Gastrointestinal toxicity-fluid or electrolyte replacements have to take Thrombocytopenia and neutropenia Tumor lysis syndrome Hepatic toxicity Embryo fetal toxicity
SIDE EFFECTS
BORTENAT 3.5MG SIDE EFFECTS
MOST COMMON SIDE EFFECTS
Black tarry stools ; Bleeding gums ; Blood in urine ; Blurred vision ; Body aches ; Burning, crawling, itching, numbness, prickling ;Chest pain ; Cough producing mucus, dizziness, faintness, nerve pain, painful urination, pale skin, runny nose, sore throat, stuffy nose, swollen glands, sunken eyes, ulcer, dry mouth, ear congestion, loss of voice
LESS COMMON SIDE EFFECTS
loss of appetite, Muscle cramps Belching, bone pain, difficulty with moving & bowel movements, cold and shivering, loss of taste, , muscle pain, stomach discomfort. Vomiting, loss of weight ; irregular breathing , Swelling of peripheral organs , Dilated veins, discomfort, increased sensitivity of pain & touch, , heart beats, , thickening of bronchial secretions
DRUG INTERACTION
Interaction with Strong CYP3A4 inhibitors then there is a chance of getting Bortenat 3.5mg toxicity; so to reduce the dose of Bortenat 3.5mg while concomitant with CYP3A4 inhibitors Strong CYP3A4 inducers: decrease the exposure of Bortenat 3.5mg Do not use combination with st. Johns wort to lowers the exposure of Bortenat 3.5mg While concomitant with melphalan-prednisone or dexamethasone alone has no clinical effect on Bortenat 3.5mg exposure
PREGNANCY
Bortenat 3.5mg is not recommended in pregnancy condition, it causes harm to fetus Breast feeding is not recommended.
LACTATION
The drug excreted into human milk is unknown and effects in the new born baby are unknown
Hence use is contraindicated
STORAGE
Bortenat 3.5mg vial should be stored at 20°C to 25°C (68°F to 77°F) excursion between 15°C to 30°C (59°F to 86°F). Keep away from light and heat.
MISSED DOSE
If a single dose missed then administer the dose as you remember, If it is near to next dose then skip the missed dose and follow the regular schedule. Do not take extra dose at a time. Consult with the doctor for further changes in dose.
| Brand name | Bortenat |
| Active substance | Bortezomib 3.5mg |
| Packaging | 1 Vial |
| Product form | Injection |
| Strength | 3.5mg |
There are no comments yet