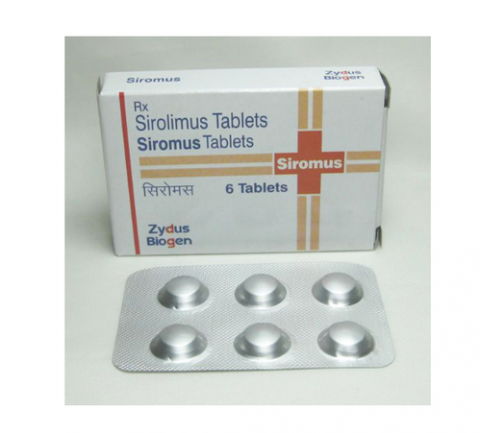
Siromus 1mg (Sirolimus )
Siromus 1mg lessen your body's immune system, to promote keep it from "deny" a transplanted organ such as a kidney.
Brand : Siromus
Ingredients : Sirolimus
Strength : 1mg
Manufactured : Zydus
Package : 6 Tablets
DESCRIPTION
Siromus 1mg lessen your body's immune system, to promote keep it from "deny" a transplanted organ such as a kidney. Organ deny happens when the immune system treats the new organ as a trespasser and attacks it.
Siromus 1mg is also taken without other medicines to cure a rare lung disorder is known as lymphangioleiomyomatosis.
Siromus 1mg which is used as prescription drug under guidance of medical practioners.
INDICATION
Siromus 1mg tablets are prescribed for the prophylaxis of organ refusal in patients aged 13 years or older diagnosed renal transplants.
MECHANISM OF ACTION
Prevents T-cell activation and replication and prevents antibody production which results in response to antigenic and cytokine stimulation; blocking T- cell multiplication by prohibiting progression from the G1 to the S phase of the cell cycle.
Lymphangioleiomyomatosis contains lung tissue infiltration with smooth muscle-like cells which inlet quiet mutations of the tuberous sclerosis complex (TSC) gene (LAM cells)
Lack of TSC gene function stimulate the mTOR signaling pathway, reporting in cellular multiplication and release of lymphangiogenic growth factors
Sirolimus prohibits the activated mTOR pathway and thus the replication of LAM cells.
ADME PROPERTY
In healthy patients and renal transplant patients the high plasma concentration is 1-2 hours and bioavailability are 27%.
The drug has plasma protein binding is 92%
Siromus is widely metabolized in the intestinal wall and liver. It is substrate for both CYP3A4 and P-gp
Most of radioactivity was recovered from the faces is 91% and only little amount was excreted in urine is 2.2%.
Half-life of Siromus is 57-63 hours.
DOSAGE MANAGEMENT
SIROMUS IS USED FOR THE PROPHYLAXIS OF RENAL TRANSPLANT REJECTION :
Starting treatment combination with cyclosporine and corticosteroids
High immunologic risk
The prescribed loading dose is <40 kg: 3 mg/m² ≥40 kg: 15 mg PO loading dose Maintenance dose of Siromus is 5 mg/day PO if >40 kg and 1 mg/m²/day if <40 kg on day 2 and after that; produce trough levels between days 5 and 7.
Dose should be adapting to maintain trough concentrations within desired range based on clinical state and combination therapy; further dose adjustment should not be done sooner than 7-14 days following a dose adjustment
Combination therapy: For the first year, continuing transplantation, Siromus should be used in interaction with cyclosporine and corticosteroids; cyclosporine may be initiated at 7 mg/kg/day in double doses with the dose modify to get trough concentrations; prednisone should be taken at a dose of 5 mg/day
Low-to-moderate immunologic risk
The loading dose <40 kg: 3 mg/m² ≥40 kg: 6 mg PO loading dose Maintenance: 2 mg/day PO if ≥ 40 k g and 1 mg/m²/day if <40 kg on day 2 and thereafter; obtain trough levels between days 5 and 7
Dose should be adapting to maintain trough concentrations within desired range based on clinical state and combination therapy; further dose adjustment should not be done sooner than 7-14 days following a dose adjustment
Combination therapy: continuing transplantation, Siromus should be used in interaction with cyclosporine and corticosteroids; may blocks cyclosporine gradually over 4-8 weeks two to four months after transplant in patients with less immunologic risk, &Siromus dose higher (serum concentrations of sirolimus may reduce following cyclosporine withdrawal)
LYMPHANGIOLEIOMYOMATOSIS :
Siromus indicated for treatment of lymphangioleiomyomatosis (LAM) Initial recommended dose: 2 mg/day PO given in 10-20 days and then monitor whole blood trough level
Over dosage :
If overdose occurs all should provide general supportive measures.
SIDE EFFECTS
Common side effects :
Fever, cold and cough
Mouth sores;
Nausea, stomach pain, diarrhoea;
Headache, muscle aches;
Chest pain;
Dizziness; or
Acne.
Other side effects, if it occurs then immediately call doctor :
Slow healing of a skin wound;
A new skin lesion,
Unusual bleeding
Sudden discomfort in chest, cough, feeling short of breath;
Pain around the transplanted kidney;
Infection like--fever, chills, painful mouth sores, skin sores, cold or flu symptoms, pain or burning when you urinate; or Anemia- skin in pale, unusual weakness, feeling light-headed or Dyspnea cold hands and feet.
PRECAUTION
Siromus 1mg should be prescribed only by physicians who have experience with immunosuppression in organ transplant recipients and can supply necessary follow-up and appropriate checking
Do not use in liver or lung transplantation as safety and efficacy is not established
In liver transplant recipients have been noticed having high mortality, loss in graft, and hepatic artery thrombosis
In lung transplant recipients has been noticed Bronchial anastomotic dehiscence
Siromus 1mg will have high risk of infection, lymphoma, and other malignancies due to raise immunosuppression
Greatest enhance sun exposure due to increased skin cancer risk
Patients generally presents with hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia will have resulted Progressive multifocal leukoencephalopathy (PML), sometimes fatal
DRUG INTERACTION
Siromus concomitant use with cyclosporine will increases sirolimus concentration. Hence sirolimus should give 4 hours after administration of cyclosporine
Avoid concomitant use of Siromus with strong inducers and strong inhibitors will decrease interaction potential with sirolimus
Using with grapefruit juice will prevents the CY3A4 – mediated metabolism of Rapacan. Avoid during using with sirolimus
CONTRAINDICATION
hypersensitivity to sirolimus
PREGNANCY
Pregnancy category is C
Siromus 1mg: There are no adequate and well-indulge studies in pregnant women. Active contraception must be started before sirolimus treatment, during sirolimus therapy, and for 12 weeks after Siromus treatment has been stopped.
LACTATION
Many drugs are excreted in human milk, and because of the potential undesirable effects in nursing infants from Siromus 1mg, a decision should be considered whether to halt for nursing or to discontinue the drug, considering the requirement of the drug to the mother.
STORAGE
Siromus 1mg Stored at 20°C-25°C
Avoid using if blister is torn or broken.
MISSED DOSE
If dose is forget to take, have it soon before next dose reaches or skip the dose and follow normal timing
Please Consult with the doctor
| Brand name | Siromus |
| Active substance | Sirolimus 1mg |
| Packaging | 6 Tablets |
| Product form | Tablet |
| Strength | 1mg |
There are no comments yet