Zyceva 100mg (Erlotinib)
Zyceva tablet has active ingredient Erlotinib, used to treat non-small cell lung cancer, pancreatic cancer and other various types of cancer. Zyceva is pharmacologically categorized as tyrosine kinase inhibitor, acts on epidermal growth factor receptor.
DESCRIPTION
Zyceva tablet has active ingredient Erlotinib, used to treat non-small cell lung cancer, pancreatic cancer and other various types of cancer. Zyceva is pharmacologically categorized as tyrosine kinase inhibitor, acts on epidermal growth factor receptor.
Zyceva tablets are prescription medicine, used only by the patients who have valid prescription
INDICATION
Zyceva is primarily indicated for; Non small cell lung cancer: Zyceva is considered as second, maintenance or greater line treatment in NSCLC. It is used after failure of some chemotherapy regimens. Zyceva is involved in the treatment of non-small cell lung cancer patients whose cancer cells containing epidermal growth factor receptors on their surface. Zyceva is not used in combination with platinum based compounds. In pancreatic carcinoma: In metastatic stage, Zyceva is used in combination with gemcitabine. In this condition Zyceva is involved as first line treatment.
PHARMACOLOGY
Zyceva has pharmacological effects like anti-tumor activity, which expels its action by prohibiting the intracellular phosphorylation of tyrosine kinase related with epidermal growth factor receptor. This EGFR is occurs on the surface of the tumor cells. Thus results as prohibition leads to intercede with signal transduction and lead to cell lyses.
ADME PROPERTY
ABSORPTION
The peak plasma concentration time reaches at 4 hours after drug intake After drug intake, the oral bioavailability of Erlotinib occurs as 60%, whereas a meal increases its bioavailability to 100%. The Erlotinib solubility is depends upon pH level. The reduction in solubility causes elevation of the pH levels. Smoking should be avoided during the treatment with Zyceva , causes decreasing the exposure of Erlotinib.
DISTRIBUTION
Volume of distribution of Erlotinib is 232L Human protein binding to Erlotinib is occurs as 93%.
METABOLISM
The metabolism of Erlotinib is occurs by CYP3A4
ELIMINATION
The route of elimination of Erlotinib metabolites occurs via; Feces: 83%; urine: 8% The terminal half life period of Erlotinib is 36.2 hours.
DOSAGE MANAGEMENT
DOSAGE MANAGEMENT
In NSCLC: The recommended dosage is 150mg should be taken as a single dose by administering on an empty stomach. In pancreatic cancer: The usual dosage of Zyceva is 100mg should be taken as a single dose by administering on an empty stomach by combining with gemcitabine. Dose alteration: In pulmonary toxicity: Interstitial lung disease, pulmonary failure: Discontinue the Zyceva therapy In severe hepatic failure: Stop the therapy permanently
In renal impairment: Discontinue the Zyceva therapy permanently In gastrointestinal perforation and severe diarrhea: Stop the therapy In severe Bullous and exfoliative skin disorders: Discontinue the therapy and provide supportive measures In corneal perforation, therapy should be discontinuing permanently. Avoid concomitant use of Zyceva with CYP3A4 strong inhibitors, CYP3A4 strong inducers, cigarette smoking, gastric regulators etc.
SIDE EFFECTS
SOME SERIOUS ADVERSE EFFECT
Renal failure Liver toxicity with failure Gastrointestinal perforations Ocular disorders Hemorrhage Bullous & exfoliative skin disorders Cerebrovascular disorders Hemolytic anemia & thrombocytopenia
SOME COMMON SIDE EFFECTS
Rash Diarrhea Cough Dyspnea Dry skin Back pain Conjunctivitis Mucosal inflammation Pruritus Paronychia Chest pain, arthralgia Musculoskeletal pain Myopathy like rhabdomyolysis while combining with lipid lowering drugs
DRUG INTERACTION
CYP3A4 INHIBITORS:
Concomitant use of Zyceva with CYP3A4 inhibitors like (boceprevir, clarithromycin, conivaptan, indinavir, itraconazole, ketaconazole, lopinavir/ritonavir, nefazodone, nelfinavir, posaconazole, ritonavir, saquinavir, telithromycin, voriconazole, grapefruit or grapefruit juice) causes increasing the exposure of Erlotinib. This result as increase in risk of adverse effects related to Zyceva .
IN CYP3A4 INDUCERS:
In combination of Zyceva with CYP3A4 inducers like anti-convulsants, rifampin, rifampicin, st Johns wort etc causes decreasing the exposure of Erlotinib. Thus results as decrease in therapeutic effect of Zyceva .
CYP1A2 INDUCERS & CIGARETTE SMOKING:
Both are decreasing the exposure of Erlotinib, while concomitant with Zyceva .
GASTRIC REGULATORS:
While concomitant with gastric regulators like proton pump inhibitors, H2 receptors antagonist or antacids, causes depletion of Erlotinib exposure leads to loss of therapeutic effect of Erlotinib.
ANTICOAGULANTS:
In combination of Zyceva with warfarin, causes increasing international normalized ratio & bleeding adverse reactions. To avoid this problem, monitor the prothrombin time frequently.
PRECAUTION
In interstitial lung disease: Severe pulmonary toxicity occurs, this may prevent by discontinue the therapy with Zyceva . Renal failure: To prevent this problem, patients should be monitored periodically with renal function test. On the other hand stop the therapy with Zyceva . Hepatic failure: Liver function test should be taken and monitored the patients frequently for any obstruction in liver functions. To avoid this complication, withhold or discontinue the therapy with Zyceva
In GI perforation: This condition occurs due to concomitant use of Zyceva with NSAIDS, taxane based chemotherapy agents, anti-angiogenic drugs, or corticosteroids etc. This is prevent by avoiding these combinations, or stop the treatment with Zyceva . Hemorrhage: To monitor the patient prothrombin time and INR during the therapy. This condition is majorly occurred due to combination of Zyceva with warfarin. Avoid this concomitant. Zyceva is not recommended in pregnancy period, this results as embryo fetal damage.
PREGNANCY & LACTATION
The pregnancy category of Erlotinib is D Zyceva tablets are not recommended for pregnancy conditions, because it may cause fetal harm even to death. Breast feeding should not be recommended.
CONTRAINDICATION
No contraindication occurs during Zyceva therapy. Some hypersensitivity reactions occur due to ingredients of Zyceva is contraindicated to the patients.
STORAGE
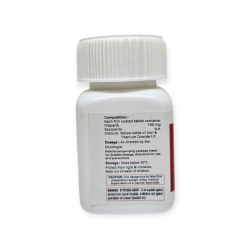
Zyceva should be stored at 25°C (77°F). Zyceva tablets should be keeping away from heat, light & moisture.
OVER DOSAGE
The over dosage of Zyceva is not possible in all conditions. If it may occurs, postpone or discontinue the Zyceva therapy and provide the patients with symptomatic therapy
MISSED DOSE
Zyceva is a chemo medicine, prescribed by medical oncologist. If patients fail to take the dose of Zyceva tablet, consult with physician and follow the instructions. On the other hand, the missed dose should be skipped and follow the regular dosing schedule.
| Brand name | Zyceva |
| Active substance | Erlotinib 100mg |
| Packaging | 30 Tablets |
| Product form | Tablet |
| Strength | 100mg |
There are no comments yet